About peptide synthesis
In organic chemistry, the process of production of peptides in which various amino acids are linked via amine bonds or peptide bonds is called peptide synthesis. In the biological process, the production of peptides or proteins is called protein biosynthesis. The techniques of linking of amino acids to the chain are the old but it will help to find out the solutions for many problems.
Applications of peptide synthesis
The invention of the peptide synthesis enhances the development of different applications, such as
- It helps to study the protein functions, identifications, and characterization of proteins.
- It helps to develop the epitope-specific antibodies against pathogenic proteins.
- It helps to study the enzyme- substrate interacts within the important enzyme classes like kinases and proteases (these play a vital role in cell signalizing).
- Synthetic peptides are used as reagents in MS (mass spectrometry) based application.
SPPS – Solid Phase Peptide Synthesis
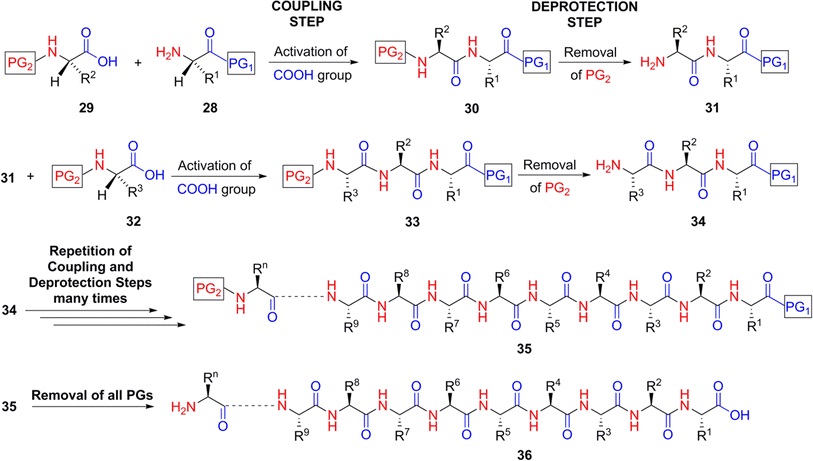
Peptide synthesis should be done on a rainin symphony peptide synthesizer or some of the similar instrumentation which is capable of automated solid-phase peptide synthesis. The expanded method for the protection of the synthetic peptides in the laboratory is called solid phase peptide synthesis. SPPS helps to make the rapid assembly of the peptide chain through the reactions of amino acid derivatives on the insoluble porous support. The nasal peptide chain linked with the reactive groups which are functionalized with the small, polymeric resin. The peptides remain covalently stick to the support throughout the peptide synthesis. You can remove only the excess reagents and side products by filtering and washing.
Each amino acid should be coupled to the peptide chain N-terminus which is protected on its N- terminal and side chain using appropriate protecting chain. Based on the side chain, the protection strategy is used.
The normal procedure of SPPS is the repeated cycles of alternate N-terminal deprotection and coupling reaction. The resin should be washed between each step. The first step is amino acid coupled to the resin. The amine is deprotected, and then it will couple with the second amino acids. This action is repeated until the required synthesis is formed. The peptide synthesis has some limitations in the range of 70 amino acids can’t be assessable for synthesis.
Improvements of SPPS
After some long years, different variants and improvements of SPPS have been developed by exchanging chemicals to optimize for certain applications. Different resins allow for various functional groups at the c-terminus. The oxymethylphenylacetamidomethly (PAM) resin and wang resin formed in the conventional c-terminal carboxylic acid. The paramethylbenzhydrylamine (pMBHA) and rink amide give the c-terminal amide which is used in mimicking the interior of a protein.
The main advantages of the improved SPPS are good acid stability and ultraviolet absorption which helps to monitor and prepare easily. Moreover, the side chains have to be protected and prevent the formation of branched chains. It should be stable, permanent and compatible with Na-protection however it is easy to remove with acid after the peptide synthesis.